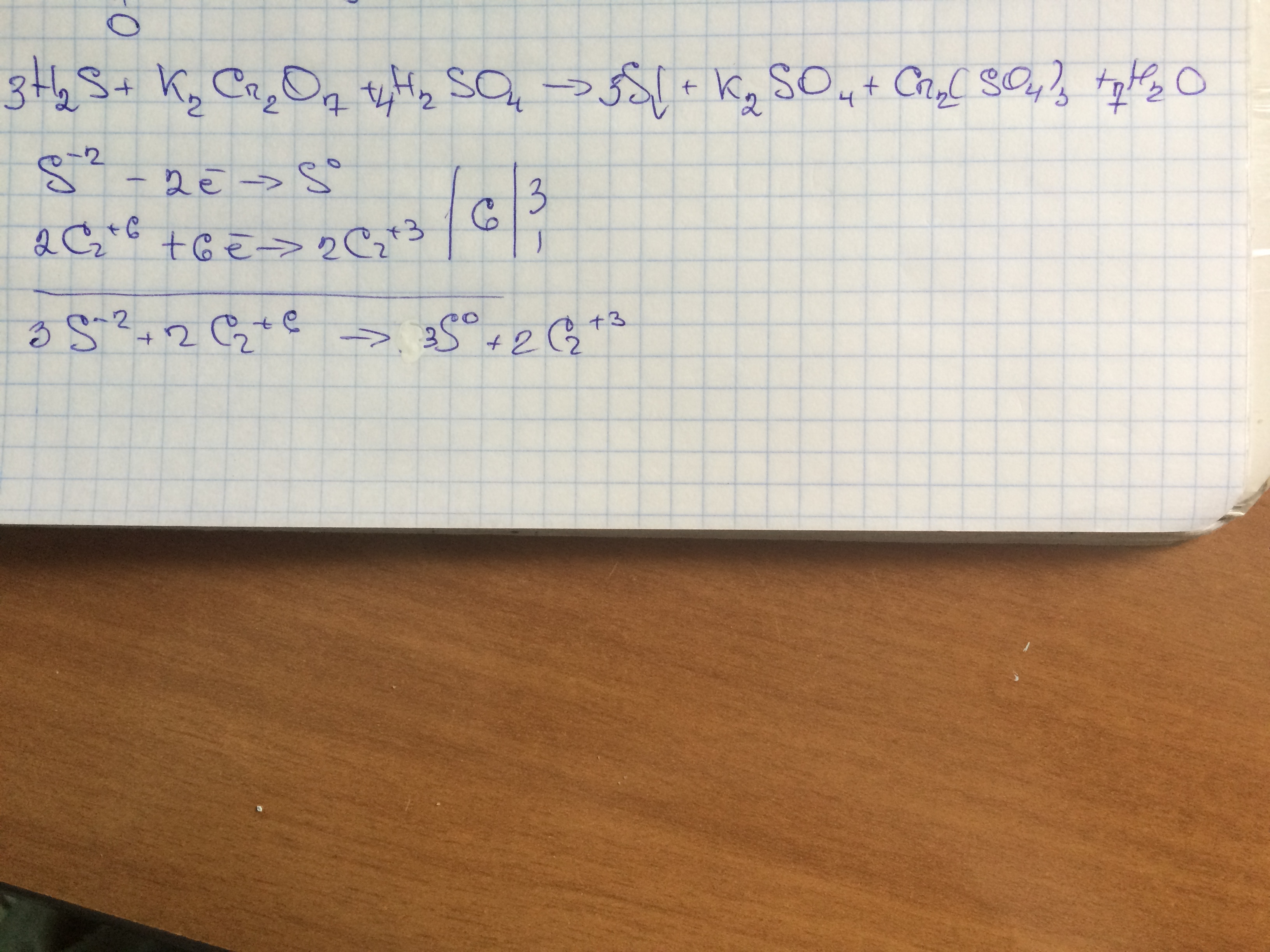Chemistry can seem intimidating, but breaking down complex reactions into smaller parts can make it easier to understand. Let's explore a chemical equation and see what each component does. We will examine the reaction involving potassium dichromate, potassium sulfite, and sulfuric acid.
The Players in Our Chemical Drama
First, let's identify each chemical compound. We have K2Cr2O7, which is potassium dichromate. Then we have K2SO3, which represents potassium sulfite. And finally, H2SO4 is sulfuric acid. These are our reactants, the ingredients that start the chemical reaction.
On the other side of the equation, we see the products formed from the reaction of our reactants. We have Cr2(SO4)3, which is chromium(III) sulfate. Next, K2SO4 is potassium sulfate. Lastly, H2O, which most of us know as plain old water.
Understanding Chemical Formulas
Let's dissect these chemical formulas to understand them better. Each formula tells us what elements are present and in what proportion. For instance, K2Cr2O7 indicates that for every molecule, we have two potassium (K) atoms, two chromium (Cr) atoms, and seven oxygen (O) atoms. Understanding the numbers, or subscripts, helps in understanding the ratios.
Similar logic applies to other formulas. H2SO4 has two hydrogen (H) atoms, one sulfur (S) atom, and four oxygen (O) atoms. The chemical formula acts as a symbolic representation of the molecular structure and composition.
The Chemical Reaction
In a chemical reaction, the reactants undergo a transformation to form products. Atoms are neither created nor destroyed but rearranged to form new compounds. The starting materials, or reactants, break chemical bonds to form new chemical bonds.
In our reaction, potassium dichromate, potassium sulfite, and sulfuric acid react to produce chromium(III) sulfate, potassium sulfate, and water. This is a redox reaction, where one substance is reduced while another is oxidized. The dichromate acts as the oxidizer here.
Real-World Applications
While this specific reaction might seem abstract, similar chemical reactions are used in various applications. Potassium dichromate has been used in cleaning glassware in laboratories and as an oxidizing agent. Sulfuric acid is a common industrial chemical used in the production of fertilizers, detergents, and various other processes.
Even the simple formation of water, a product of this reaction, is a fundamental process that occurs in many chemical and biological systems. The understanding of these reactions becomes critical in diverse fields like medicine, engineering, and environmental science.
In Summary
Chemical equations can seem daunting, but understanding each part makes them much easier to grasp. We've explored the reactants and products of a specific chemical reaction and learned the importance of chemical formulas. Remember to take chemical equations one step at a time, and you will master them in no time!
3+%2B+I2+%2B+H2O+%2B+Na2SO4+%2B+K2SO4.jpg)

.ppt_images/1-upraghneniya_po_permanganatu_i_dihromatu_(2).ppt_5.jpg)




3+%2B+I2+%2B+H2O+%2B+Na2SO4+%2B+K2SO4.jpg)
3+%2B+K2SO4+%2B+H2O+%EF%83%A0+3PbSO4+%2B+K2Cr2O7+%2B+H2SO4.jpg)