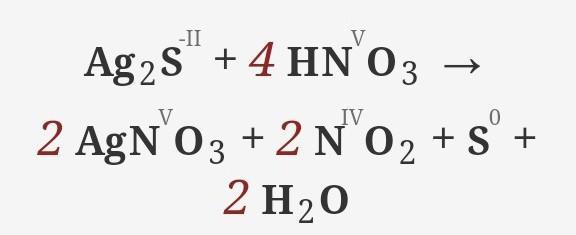
Hej! Przygotowujesz się do egzaminu z chemii? Świetnie! Razem to ogarniemy. Skupimy się na reakcji: Ag2S + HNO3 → AgNO3 + NO + S + H2O.
Reakcja Redox: Podstawy
Na początek, przypomnijmy sobie czym jest reakcja redox. To reakcja, w której zachodzi przekaz elektronów pomiędzy reagentami. Jedna substancja się utlenia (traci elektrony), a druga redukuje (zyskuje elektrony).
Co trzeba wiedzieć?
Musisz umieć określać stopnie utlenienia pierwiastków w związkach chemicznych. Pamiętaj o regułach! Na przykład, tlen zazwyczaj ma stopień utlenienia -2, a wodór +1.
Musisz też wiedzieć, co to jest utleniacz i reduktor. Utleniacz to substancja, która przyjmuje elektrony (redukuje się). Reduktor to substancja, która oddaje elektrony (utlenia się).
Analiza Reakcji Ag2S + HNO3
Spójrzmy na naszą reakcję: Ag2S + HNO3 → AgNO3 + NO + S + H2O.
Określanie Stopni Utlenienia
Zacznijmy od określenia stopni utlenienia pierwiastków przed i po reakcji.
- Ag2S: Srebro (Ag) ma stopień utlenienia +I, a siarka (S) -II.
- HNO3: Wodór (H) ma stopień utlenienia +I, azot (N) +V, a tlen (O) -II.
- AgNO3: Srebro (Ag) ma stopień utlenienia +I, azot (N) +V, a tlen (O) -II.
- NO: Azot (N) ma stopień utlenienia +II, a tlen (O) -II.
- S: Siarka (S) ma stopień utlenienia 0.
- H2O: Wodór (H) ma stopień utlenienia +I, a tlen (O) -II.
Co się zmieniło?
Zauważ, że stopień utlenienia azotu (N) zmienił się z +V w HNO3 na +II w NO. A stopień utlenienia siarki (S) zmienił się z -II w Ag2S na 0 w S.
Identyfikacja Utleniacza i Reduktora
Skoro stopień utlenienia azotu zmalał, to znaczy, że azot się redukuje. Zatem HNO3 jest utleniaczem.
Skoro stopień utlenienia siarki wzrósł, to znaczy, że siarka się utlenia. Zatem Ag2S jest reduktorem.
Bilans Elektronowy
Teraz najważniejsze – bilans elektronowy! To klucz do poprawnego zbilansowania równania reakcji redox.
Półreakcje
Rozpisujemy półreakcje utleniania i redukcji:
- Utlenianie: S-II → S0 + 2e-
- Redukcja: N+V + 3e- → N+II
Wyrównanie Elektronów
Musimy wyrównać liczbę elektronów oddanych i przyjętych. Najmniejsza wspólna wielokrotność 2 i 3 to 6.
- Mnożymy półreakcję utleniania przez 3: 3S-II → 3S0 + 6e-
- Mnożymy półreakcję redukcji przez 2: 2N+V + 6e- → 2N+II
Zbilansowane Równanie
Teraz możemy zbilansować całe równanie reakcji, używając współczynników z bilansu elektronowego.
Zaczynamy od siarki (S) i azotu (N):
Ag2S + HNO3 → AgNO3 + 2NO + 3S + H2O
Przed siarką w produktach wpisujemy 3, a przed NO wpisujemy 2. Teraz bilansujemy srebro (Ag):
3Ag2S + HNO3 → 6AgNO3 + 2NO + 3S + H2O
Przed AgNO3 wpisujemy 6, bo mamy 3 * 2 = 6 atomów srebra w substratach. Teraz bilansujemy azot (N):
3Ag2S + 8HNO3 → 6AgNO3 + 2NO + 3S + H2O
Po stronie produktów mamy 6 atomów azotu w AgNO3 i 2 atomy azotu w NO, czyli razem 8. Zatem przed HNO3 wpisujemy 8. Na koniec bilansujemy wodór (H) i tlen (O):
3Ag2S + 8HNO3 → 6AgNO3 + 2NO + 3S + 4H2O
Przed H2O wpisujemy 4, bo mamy 8 atomów wodoru w HNO3. Sprawdzamy tlen: po stronie substratów mamy 8 * 3 = 24 atomy tlenu, a po stronie produktów 6 * 3 + 2 + 4 = 18 + 2 + 4 = 24 atomy tlenu. Zatem równanie jest zbilansowane!
Ostateczne Zbilansowane Równanie
Oto ostateczne, zbilansowane równanie reakcji:
3Ag2S + 8HNO3 → 6AgNO3 + 2NO + 3S + 4H2O
Podsumowanie
- Reakcja redox to reakcja z przekazywaniem elektronów.
- Musisz umieć określać stopnie utlenienia.
- Utleniacz redukuje się (przyjmuje elektrony).
- Reduktor utlenia się (oddaje elektrony).
- Bilans elektronowy jest kluczowy do zbilansowania równania.
- Rozpisuj półreakcje utleniania i redukcji.
Pamiętaj, ćwiczenie czyni mistrza! Rozwiązuj zadania, analizuj różne reakcje i nie bój się pytać. Powodzenia na egzaminie!