Converting 13.6 g/cm³ to kg/m³ might seem daunting at first. It is a common task in physics and chemistry. Educators can simplify this process.
Understanding the Units
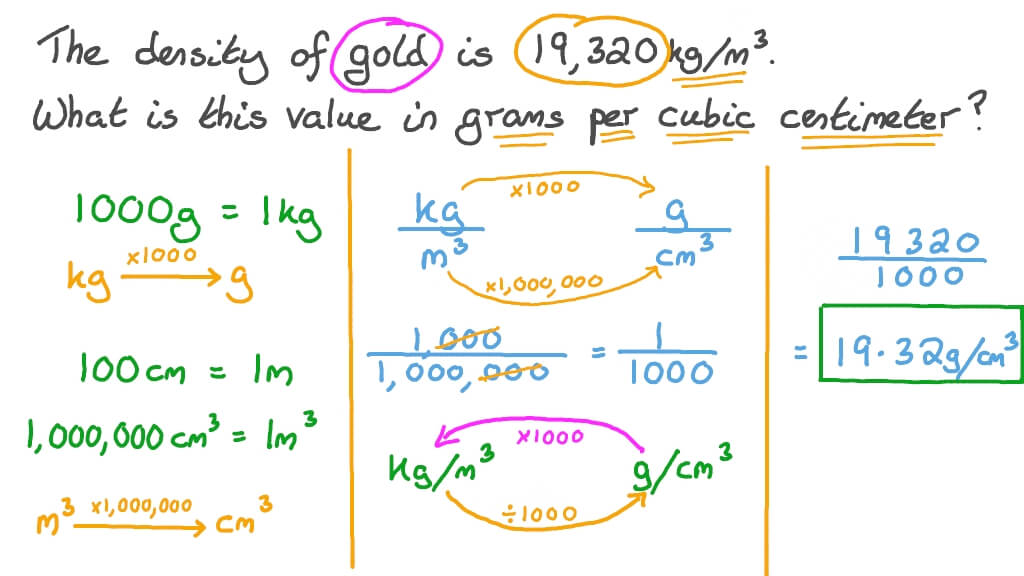
Let’s examine the units involved. We have grams per cubic centimeter (g/cm³) and kilograms per cubic meter (kg/m³). These units represent density. It's the mass per unit volume.
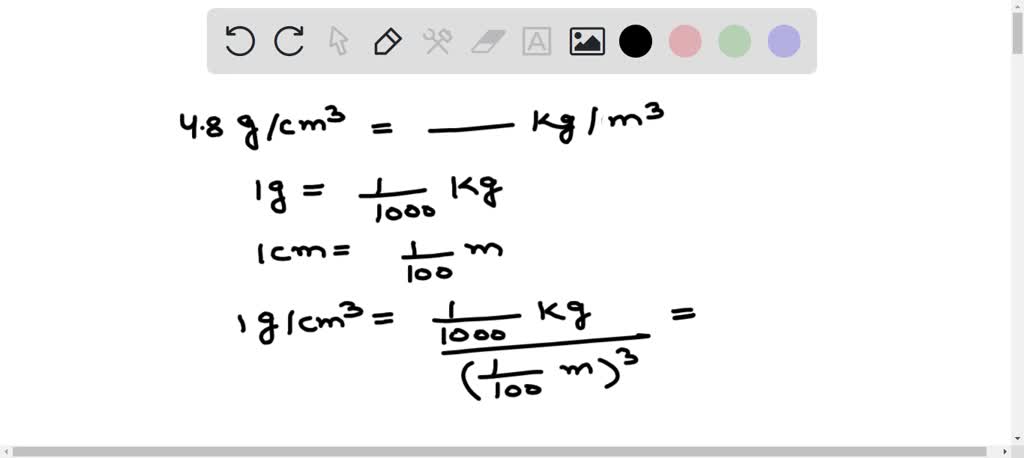
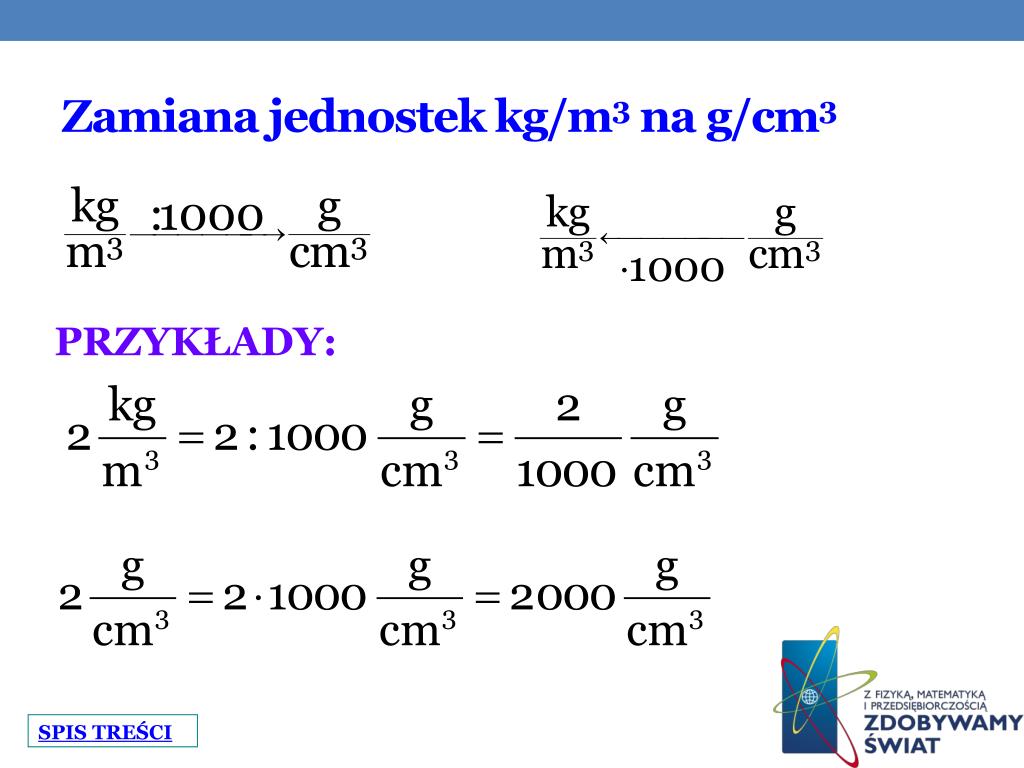
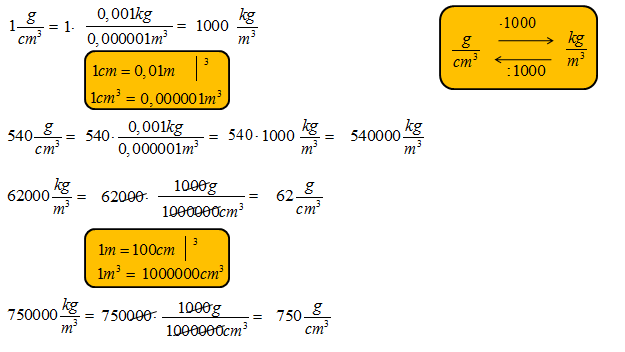
To convert, remember the relationships between the units. 1 kg equals 1000 g. 1 m equals 100 cm.
The Conversion Process
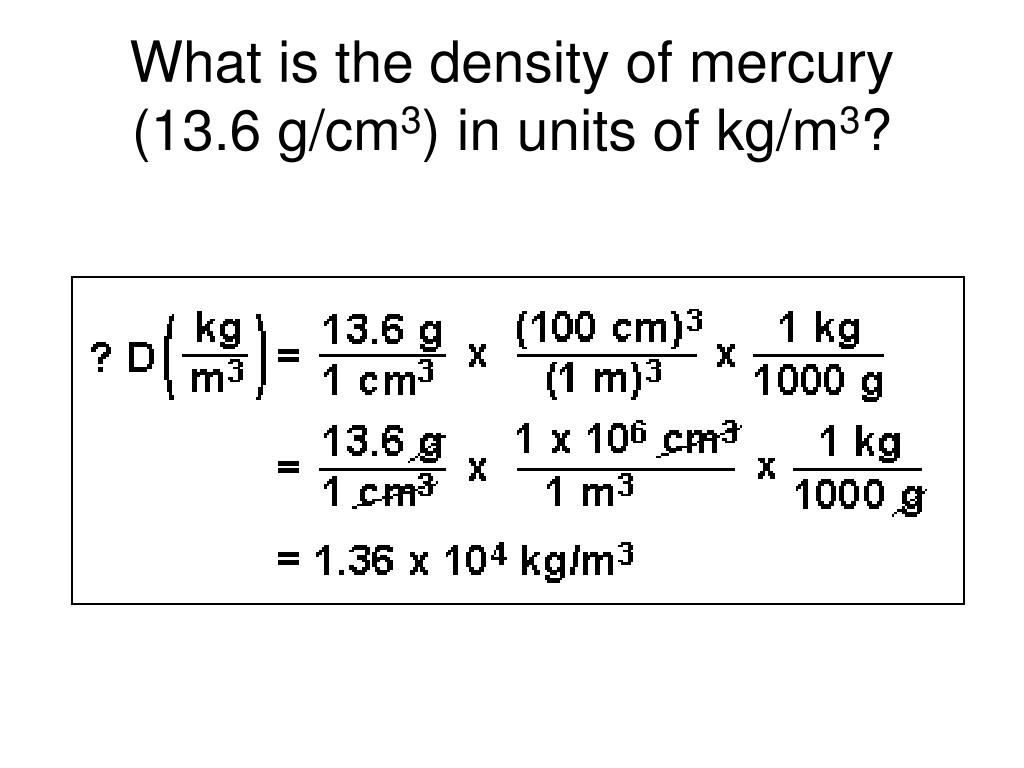
Start with the initial value: 13.6 g/cm³. We need to convert grams to kilograms. We also convert cubic centimeters to cubic meters.
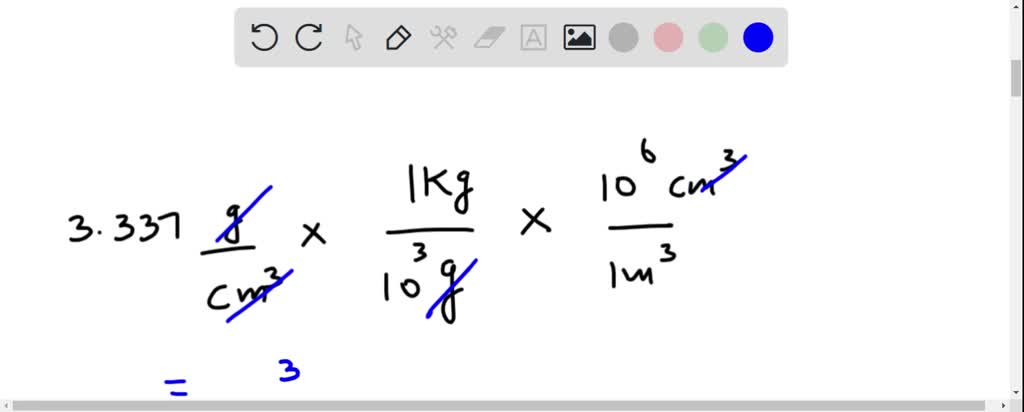
First, convert grams to kilograms. Divide 13.6 g by 1000. This gives us 0.0136 kg/cm³.
Now, convert cm³ to m³. Since 1 m = 100 cm, then 1 m³ = (100 cm)³ = 1,000,000 cm³. Multiply 0.0136 kg/cm³ by 1,000,000. The result is 13,600 kg/m³.
Simplified Explanation for Students
Explain that we're changing the scale of measurement. Imagine zooming out from centimeters to meters. The volume increases significantly.
Use relatable examples. Compare the density of water in g/cm³ and kg/m³. This provides a tangible context.
Show the conversion factors clearly. Emphasize the relationship between grams and kilograms. Also show the relationship between cubic centimeters and cubic meters.
Common Misconceptions
A common mistake is forgetting to cube the conversion factor for volume. Students often only convert centimeters to meters. They don't cube the result to get cubic meters.
Some students struggle with the concept of density. They may confuse it with mass or volume. Reinforce the definition of density: mass per unit volume.
Another misconception is not understanding unit cancellation. Demonstrate how units cancel out during the conversion. This reinforces the logic of the process.
Engaging Activities
Conduct experiments to measure the density of different objects. Use both grams and kilograms. Also, use both cubic centimeters and cubic meters. This hands-on approach solidifies understanding.
Use online simulations to visualize the conversion. There are tools available that demonstrate the conversion. Students can input values and see the results instantly.
Create a game or a competition involving unit conversions. Reward accurate and quick conversions. This makes learning fun and competitive.
Tips for Educators
Always emphasize the importance of units in calculations. Remind students that incorrect units lead to wrong answers. Encourage students to include units in every step of their calculations.
Break down the conversion into smaller, manageable steps. This makes the process less intimidating. Provide ample practice problems.
Use visual aids such as diagrams and charts. These help students understand the relationships between the units. These charts can show the conversion process visually.
Connect the concept to real-world applications. Discuss how density is used in engineering and materials science. This adds relevance to the topic.
By using these strategies, educators can effectively teach the conversion. Students will then better understand the relationship between 13.6 g/cm³ and 13600 kg/m³.
Remember to be patient and supportive. Unit conversions can be challenging. Consistent practice and clear explanations are key.