Hey there! Let's dive into a fascinating topic: the radius of the first orbit in a hydrogen atom, often called the Bohr radius. It might sound complicated, but we will break it down step-by-step. We'll explore what it is, why it's important, and how it's calculated. Buckle up, it will be an exciting ride!
What's an Atom Anyway?
Before we talk about the hydrogen atom and its radius, let's quickly review what an atom is. Think of it as the smallest building block of matter. Everything around us, from the air we breathe to the phone you're reading this on, is made up of atoms. Each atom contains a central core called the nucleus. The nucleus is made of positively charged particles called protons and neutral particles called neutrons.
Circling the nucleus are negatively charged particles called electrons. These electrons orbit the nucleus in specific paths, like planets orbiting the sun. These paths are called energy levels or electron shells. Think of it like a multi-lane highway around a city – electrons can only travel on these specific lanes, not in between.
Hydrogen: The Simplest Atom
Hydrogen is the simplest of all atoms. It has only one proton in its nucleus and one electron orbiting it. This makes it a perfect starting point for understanding atomic structure. Because it is so simple, it allows us to describe it precisely. It serves as a cornerstone to understanding complex atoms.
Imagine hydrogen as a tiny solar system with a single "sun" (the proton) and a single "planet" (the electron). The electron is held in orbit around the proton because of the electrostatic force of attraction between opposite charges. Just like gravity keeps planets orbiting the sun, this electric force keeps the electron bound to the atom.
Introducing the Bohr Model
To understand the radius of the first orbit, we need to know about the Bohr model. This model, developed by Niels Bohr in 1913, was a groundbreaking attempt to explain the structure of the atom. It proposed that electrons could only exist in specific energy levels or orbits around the nucleus.
These orbits are quantized, meaning that the electron can only occupy certain allowed distances from the nucleus. It's like a staircase – you can stand on one step or another, but not in between. The Bohr model brilliantly explained the observed spectrum of hydrogen, which classical physics could not.
The First Orbit and Its Radius
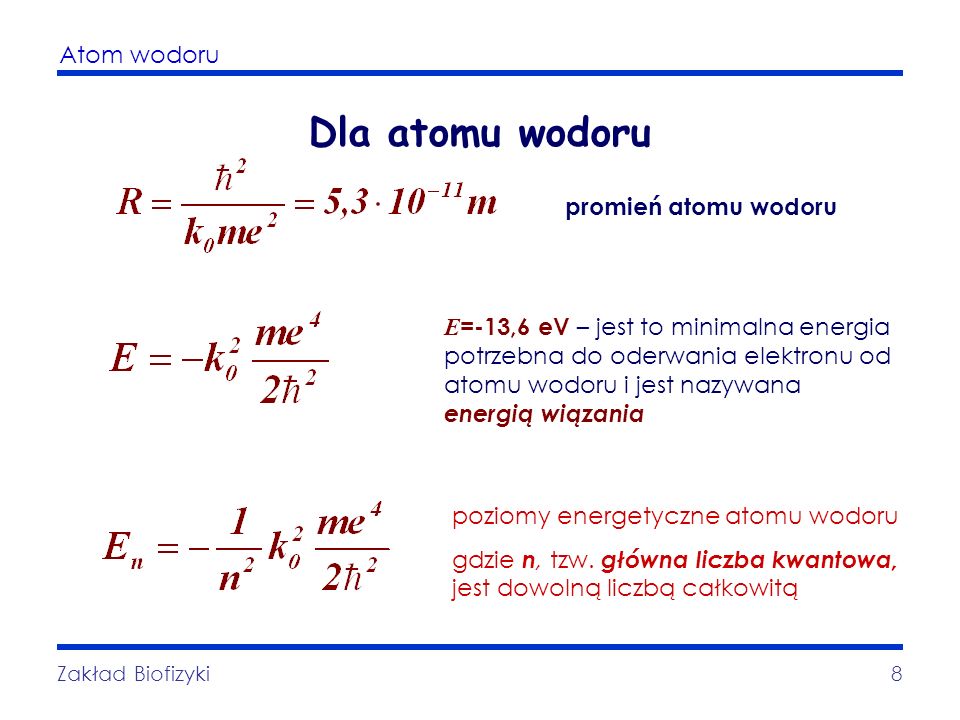
The first orbit, also known as the ground state, is the orbit closest to the nucleus. It's the orbit with the lowest energy. The Bohr radius (denoted as a₀) is the radius of this first orbit in a hydrogen atom. It is a fundamental constant in atomic physics.
So, what is the value of the Bohr radius? It's approximately 5.29 x 10-11 meters, or 0.0529 nanometers. This is incredibly small! To put it in perspective, if you magnified a hydrogen atom to the size of a football field, the Bohr radius would be about the width of a human hair.
Why is the Bohr Radius Important?
The Bohr radius is essential because it gives us a sense of the size of atoms. It is a fundamental unit of length in atomic physics. It is like a yardstick for measuring atomic distances. Understanding the Bohr radius is crucial for calculating various atomic properties. It helps predict how atoms will interact with each other.
It's also a key component in many calculations involving atomic and molecular structure. It's used as a scaling factor for atomic units, which simplifies calculations. It also helps scientists to understand chemical bonding, spectroscopy, and many other aspects of chemistry and physics.
Calculating the Bohr Radius
So, how do we calculate the Bohr radius? It's derived from fundamental physical constants using the following formula:
a₀ = ħ² / (mₑ * e² * k)
Where:
- a₀ is the Bohr radius
- ħ (h-bar) is the reduced Planck constant (Planck constant divided by 2π), approximately 1.054 x 10-34 J⋅s
- mₑ is the mass of the electron, approximately 9.109 x 10-31 kg
- e is the elementary charge, the charge of a proton or an electron (magnitude), approximately 1.602 x 10-19 C
- k is Coulomb's constant, approximately 8.987 x 109 N⋅m²/C²
Don't worry if these symbols look intimidating! It's just a combination of numbers that nature has given us. If you plug in these values into the formula, you'll get approximately 5.29 x 10-11 meters, which confirms the Bohr radius.
Beyond the Bohr Model
It's important to remember that the Bohr model is a simplified picture of the atom. While it accurately predicts the radius and energy levels of hydrogen, it doesn't work as well for atoms with more than one electron. More sophisticated models, like the quantum mechanical model, are needed to describe complex atoms accurately.
The quantum mechanical model describes electrons not as orbiting in definite paths but as existing in regions of space called atomic orbitals. These orbitals are probability distributions that describe the likelihood of finding an electron at a particular location around the nucleus. This model has greater accuracy and allows us to understand other atoms.
Conclusion
So, there you have it! The radius of the first orbit in a hydrogen atom, the Bohr radius, is a fundamental constant that helps us understand the size and structure of atoms. While the Bohr model is a simplified picture, it provides a valuable foundation for understanding more complex atomic models. With this foundation you can understand the structure of all matter around us.
Keep exploring, keep learning, and you'll be amazed by the wonders of the atomic world! Remember the Bohr radius (5.29 x 10-11 meters) as the foundational length scale for everything else in nature.