Wyobraź sobie, że masz konkurs tańca. Na parkiecie jest miedź (Cu) i azotan srebra (AgNO3).
Miedź bardzo lubi tańczyć. Azotan srebra to tak naprawdę połączenie srebra (Ag) i azotanu (NO3).
Srebro też chce tańczyć, ale jest przywiązane do azotanu. Tańczą razem, ale niezbyt szczęśliwie.
Reakcja Redox: Taniec Zamiany
Nadchodzi moment, gdy miedź wkracza na parkiet. Jest pewna siebie i pełna energii!
Miedź jest jak ktoś, kto ma więcej siły i chęci do tańca niż srebro. Chce przejąć partnera!
To jest właśnie reakcja redoks! To jak zamiana partnerów w tańcu, gdzie jeden oddaje, a drugi przyjmuje.
Utlenianie: Miedź Oddaje Elektrony
Miedź, by wejść w "taniec" z azotanem, musi oddać coś od siebie. Oddaje dwa elektrony.
Pomyśl o elektronach jak o wizach wstępu na ekskluzywny parkiet. Miedź oddaje swoje wizy.
Oddawanie elektronów nazywamy utlenianiem. Miedź się utlenia. Staje się jonem miedzi (Cu2+).
Teraz miedź jest naładowana pozytywnie (Cu2+). Jest gotowa na nowy związek!
Redukcja: Srebro Przyjmuje Elektrony
Srebro, które było związane z azotanem, dostaje te elektrony od miedzi.
Przyjmowanie elektronów nazywamy redukcją. Srebro się redukuje. Z jonu srebra (Ag+) staje się zwykłym, metalicznym srebrem (Ag).
Dzięki temu, że srebro przyjęło elektrony, przestaje być jonem i może opuścić azotan.
Wyobraź sobie, że srebro, po przyjęciu "wizy", odzyskuje swoją niezależność i opuszcza imprezę.
Co Się Dzieje Dalej?
Miedź (Cu2+) łączy się z azotanem (NO3-). Powstaje azotan miedzi(II) (Cu(NO3)2). To nowy taniec!
Srebro (Ag), uwolnione od azotanu, wytrąca się z roztworu. Osadza się na dnie naczynia.
Widzisz błyszczące, metaliczne srebro! To dowód na to, że reakcja zaszła.
Podsumowanie Reakcji
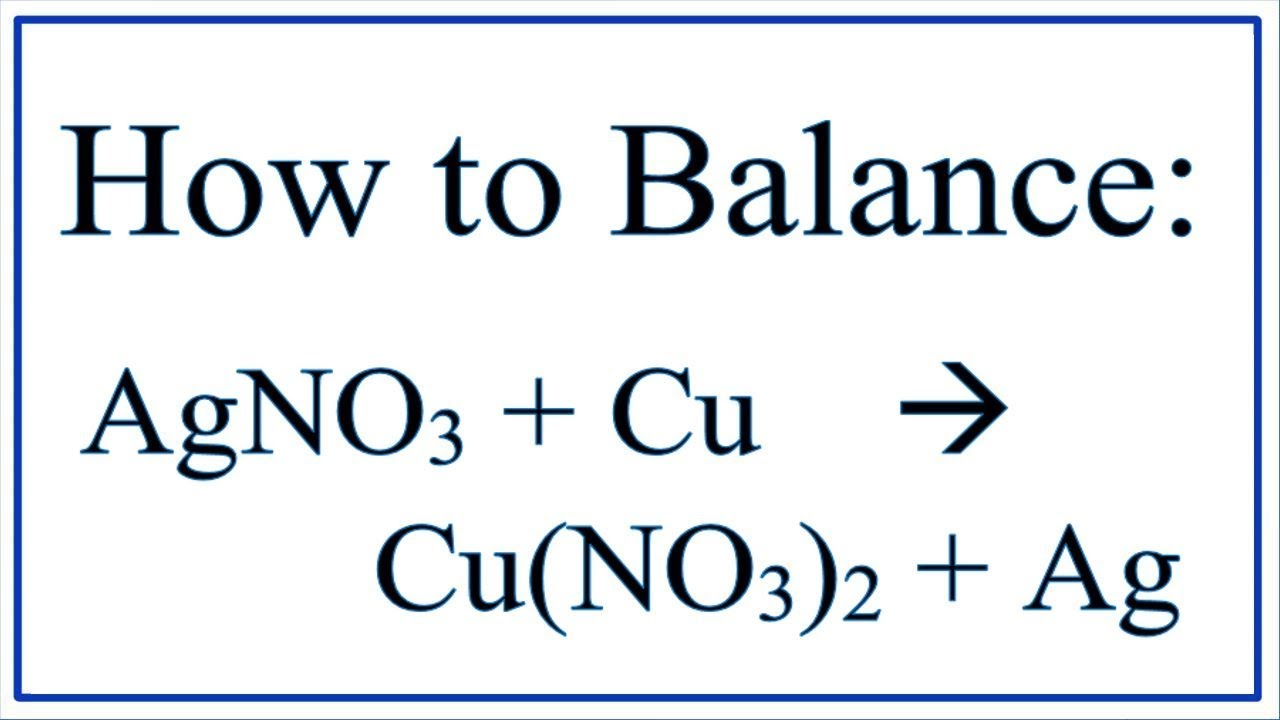
Zaczynamy od: miedź (Cu) i azotan srebra (AgNO3).
Kończymy na: azotan miedzi(II) (Cu(NO3)2) i srebro (Ag).
Równanie reakcji: Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
Pamiętaj, że miedź oddaje elektrony (utlenianie), a srebro je przyjmuje (redukcja).
Realne Przyklady
Ten rodzaj reakcji wykorzystuje się np. w procesie oczyszczania metali.
Można to też zaobserwować w prostym eksperymencie w domu. Włóż miedziany drucik do roztworu azotanu srebra.
Zauważysz, że drucik zacznie ciemnieć, a na jego powierzchni pojawi się błyszczący osad srebra!
Roztwór zmieni kolor na niebieski, co oznacza obecność jonów miedzi.
Zapamiętaj
Utlenianie to oddawanie elektronów. Redukcja to przyjmowanie elektronów.
Zawsze, gdy zachodzi utlenianie, musi zachodzić też redukcja. To dwa procesy, które są ze sobą nierozerwalnie związane.
Miedź oddaje, srebro przyjmuje. Taniec zamiany elektronów. Reakcja redoks!