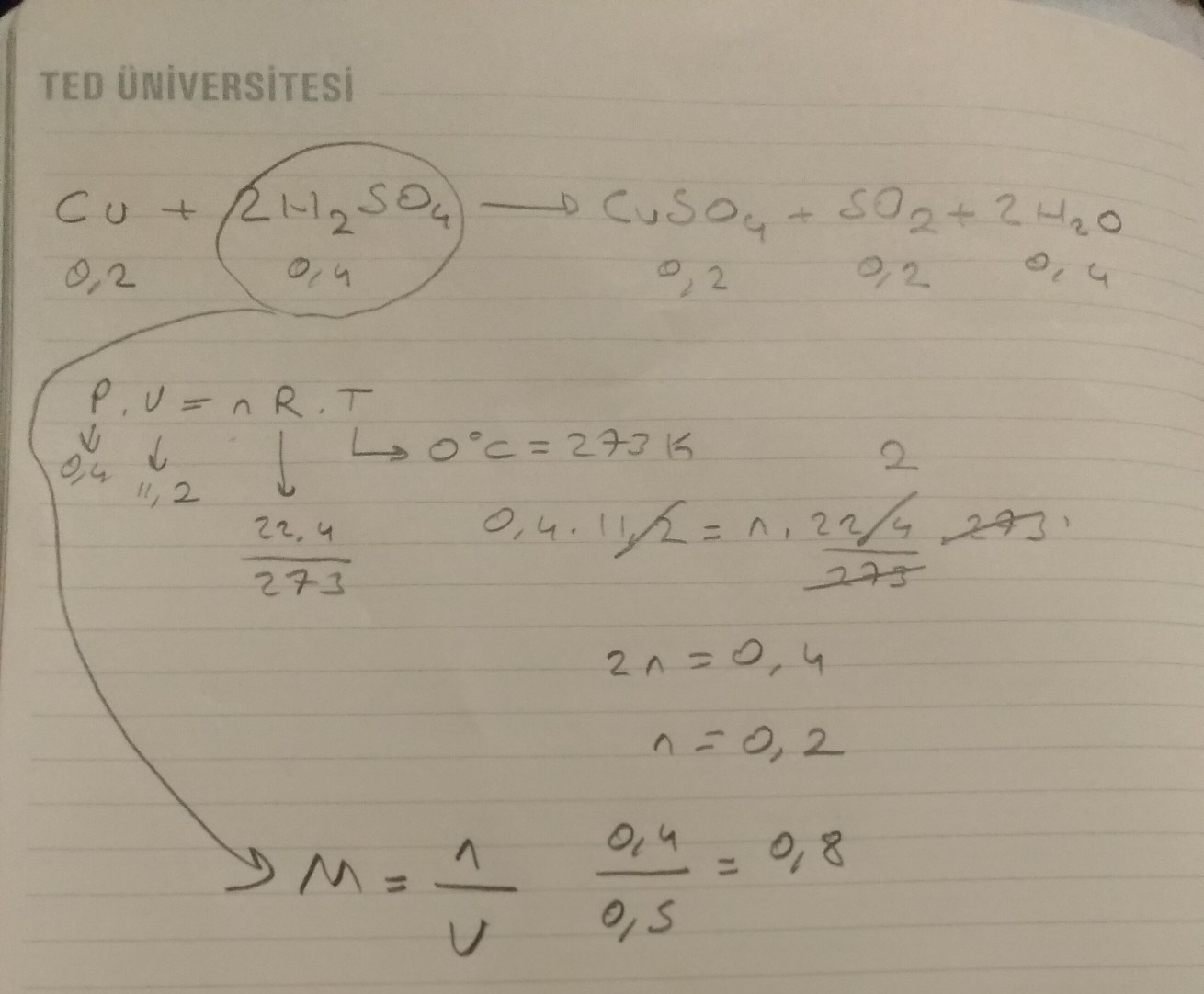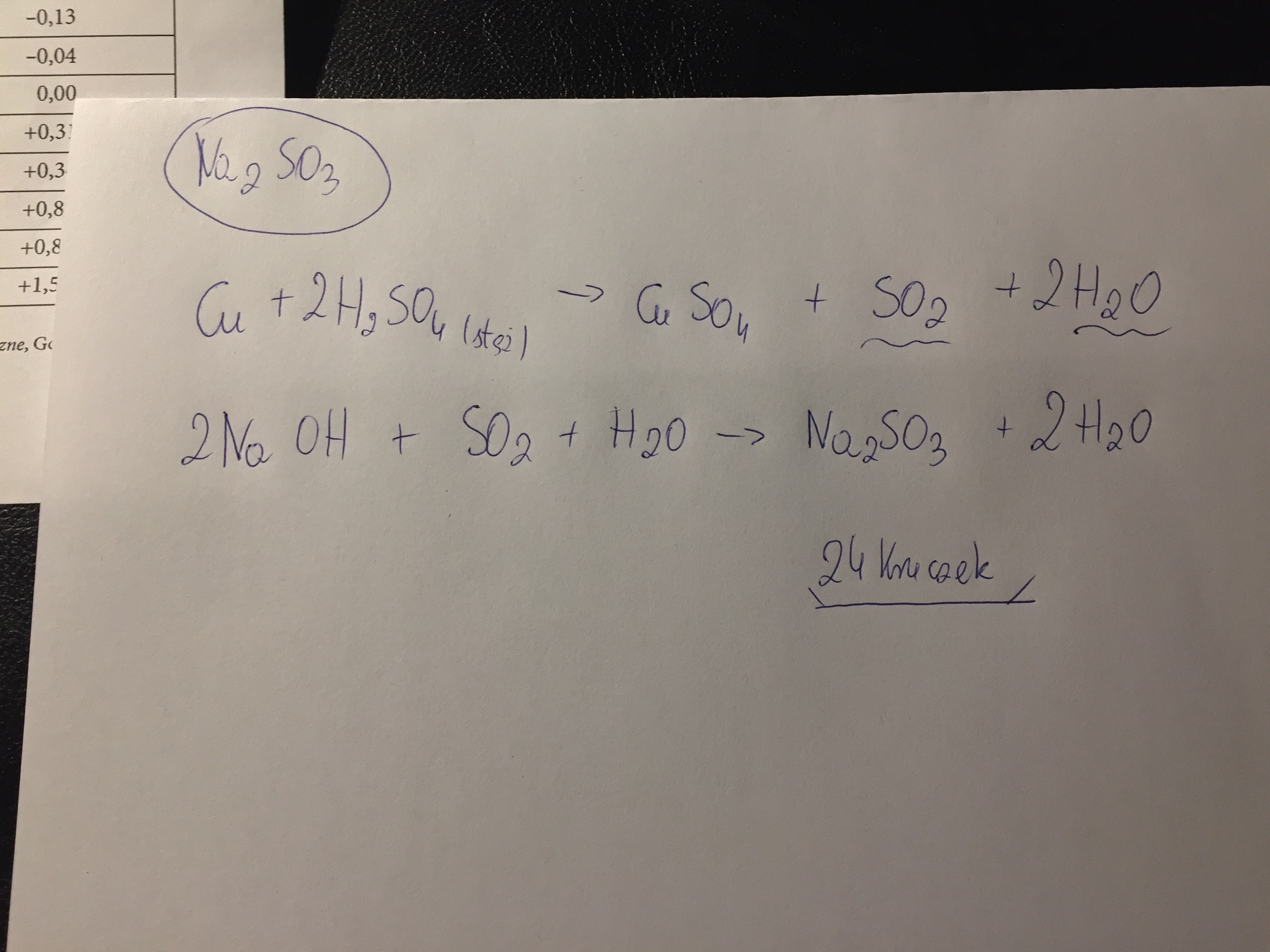Cześć! Przygotujmy się razem do egzaminu z chemii. Dziś omówimy reakcję chemiczną z udziałem Cu, H2SO4, CuSO4, SO2 i H2O.
Reakcja Miedzi z Kwasem Siarkowym
Zaczynamy od reakcji miedzi z kwasem siarkowym. Pamiętaj, że mamy dwa rodzaje kwasu siarkowego: rozcieńczony i stężony.
Miedź i Rozcieńczony Kwas Siarkowy
Miedź nie reaguje z rozcieńczonym kwasem siarkowym. Zapamiętaj to! Reakcja *nie zachodzi*.
Dlaczego? Potencjał standardowy miedzi jest wyższy niż jonów wodorowych.
Miedź i Stężony Kwas Siarkowy
Miedź reaguje ze stężonym kwasem siarkowym na gorąco. Ta reakcja jest bardzo ważna.
Równanie reakcji to: Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O.
Co powstaje? Powstaje siarczan(VI) miedzi(II) (CuSO4), dwutlenek siarki (SO2) i woda (H2O).
Omówienie Produktów Reakcji
Siarczan(VI) Miedzi(II) (CuSO4)
CuSO4 to sól. Ma charakterystyczny niebieski kolor w formie hydratu.
Hydrat to związek chemiczny zawierający wodę w swojej strukturze krystalicznej. Na przykład CuSO4·5H2O to pentahydrat siarczanu(VI) miedzi(II).
Bezwodny CuSO4 jest biały. Wykorzystuje się go do wykrywania obecności wody.
Dwutlenek Siarki (SO2)
SO2 to bezbarwny gaz o ostrym, duszącym zapachu. Jest toksyczny.
SO2 jest przyczyną kwaśnych deszczy. Powstaje w wyniku spalania paliw zawierających siarkę.
Ma właściwości redukujące i utleniające. Zależy to od warunków reakcji.
Stosowany jest jako konserwant w przemyśle spożywczym (E220).
Woda (H2O)
Woda, H2O, to produkt reakcji. Jest rozpuszczalnikiem dla soli.
Woda pełni kluczową rolę w wielu procesach chemicznych i biologicznych.
Stopnie Utlenienia
Określmy stopnie utlenienia w reakcji:
Cu0 + H2+1S+6O4-2 → Cu+2S+6O4-2 + S+4O2-2 + H2+1O-2
Miedź utlenia się ze stopnia 0 do +2. Siarka redukuje się ze stopnia +6 do +4.
Proces jest reakcją redoks (utleniania-redukcji). Miedź jest reduktorem, a kwas siarkowy utleniaczem.
Ważne Zastosowania
Reakcja ta ma znaczenie w przemyśle chemicznym. Służy do otrzymywania CuSO4 i SO2.
CuSO4 jest stosowany w rolnictwie jako fungicyd (środek grzybobójczy).
SO2 jest używany w produkcji kwasu siarkowego.
Podsumowanie
- Miedź nie reaguje z rozcieńczonym kwasem siarkowym.
- Miedź reaguje ze stężonym kwasem siarkowym na gorąco: Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O.
- CuSO4 to siarczan(VI) miedzi(II). Ma niebieski kolor (hydrat).
- SO2 to dwutlenek siarki. Jest toksyczny gaz.
- Reakcja jest reakcją redoks.
Pamiętaj! Powtórz to kilka razy, a na pewno zdasz egzamin. Powodzenia!