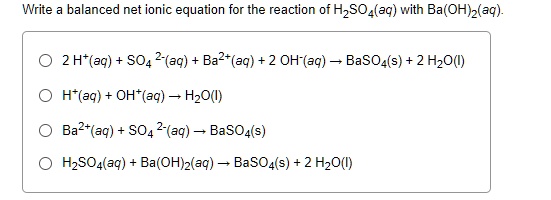Rozważmy reakcję chemiczną między wodorotlenkiem baru (Ba(OH)₂) a kwasem siarkowym (H₂SO₄). Jest to przykład reakcji zobojętniania, gdzie zasada reaguje z kwasem.
Zapiszmy najpierw równanie cząsteczkowe tej reakcji. Pokazuje ono całe cząsteczki zaangażowane w reakcję.
Ba(OH)₂ (aq) + H₂SO₄ (aq) → BaSO₄ (s) + 2H₂O (l)
Równanie Jonowe Pełne
Równanie jonowe pełne pokazuje wszystkie jony obecne w roztworze przed i po reakcji. Zauważ, że związki jonowe i mocne kwasy, które są rozpuszczalne w wodzie, dysocjują na jony. Związki nierozpuszczalne (osady) i ciecze (jak woda) pozostają w formie cząsteczkowej.
Wodorotlenek baru (Ba(OH)₂) jest mocną zasadą i rozpuszcza się w wodzie, dysocjując na jony baru (Ba²⁺) i jony wodorotlenkowe (OH⁻). Z kolei kwas siarkowy (H₂SO₄) jest mocnym kwasem (przy pierwszej dysocjacji protonu) i dysocjuje na jony wodorowe (H⁺) i jony siarczanowe (SO₄²⁻).
Siarczan baru (BaSO₄) jest nierozpuszczalny w wodzie, więc występuje w postaci stałej. Woda (H₂O) pozostaje w postaci cieczy.
Zatem, równanie jonowe pełne wygląda następująco:
Ba²⁺(aq) + 2OH⁻(aq) + 2H⁺(aq) + SO₄²⁻(aq) → BaSO₄(s) + 2H₂O(l)
Równanie Jonowe Skrócone
Równanie jonowe skrócone pokazuje tylko te jony i cząsteczki, które faktycznie biorą udział w reakcji. Eliminuje jony "widzów", czyli te, które występują po obu stronach równania w niezmienionej formie. Te jony nie biorą udziału w tworzeniu osadu lub innych produktów. Zanim zapiszemy równanie jonowe skrócone musimy zidentyfikować jony, które nie ulegają zmianie w trakcie reakcji.
W naszym przypadku, jony baru (Ba²⁺) i jony siarczanowe (SO₄²⁻) łączą się tworząc nierozpuszczalny osad siarczanu baru (BaSO₄). Jony wodorowe (H⁺) i jony wodorotlenkowe (OH⁻) łączą się tworząc wodę (H₂O).
Jony, które występują po obu stronach równania jonowego pełnego i nie biorą udziału w tworzeniu osadu lub wody to jony "widzowie". W naszym przypadku, nie ma jonów widzów. Wszystkie jony uczestniczą w tworzeniu nowych substancji.
Zatem równanie jonowe skrócone wygląda następująco:
Ba²⁺(aq) + 2OH⁻(aq) + 2H⁺(aq) + SO₄²⁻(aq) → BaSO₄(s) + 2H₂O(l)
Wyjaśnienie Krok po Kroku
Krok 1: Zapisz równanie cząsteczkowe. Upewnij się, że równanie jest zbilansowane. To daje ogólny obraz reakcji.
Ba(OH)₂ (aq) + H₂SO₄ (aq) → BaSO₄ (s) + 2H₂O (l)
Krok 2: Zapisz równanie jonowe pełne. Rozbij wszystkie rozpuszczalne związki jonowe i mocne kwasy na ich jony. Pamiętaj, że związki nierozpuszczalne, słabe elektrolity i ciecze pozostają w formie cząsteczkowej.
Ba²⁺(aq) + 2OH⁻(aq) + 2H⁺(aq) + SO₄²⁻(aq) → BaSO₄(s) + 2H₂O(l)
Krok 3: Zidentyfikuj i wykreśl jony widzów. To jony, które występują po obu stronach równania w niezmienionej formie.
W tym konkretnym przypadku nie mamy jonów widzów. Wszystkie jony biorą udział w tworzeniu nowych substancji: osadu siarczanu baru i wody.
Krok 4: Zapisz równanie jonowe skrócone. To równanie zawiera tylko te jony i cząsteczki, które faktycznie biorą udział w reakcji.
Ba²⁺(aq) + 2OH⁻(aq) + 2H⁺(aq) + SO₄²⁻(aq) → BaSO₄(s) + 2H₂O(l)
Przykłady i Aplikacje
Reakcje zobojętniania, takie jak ta pomiędzy wodorotlenkiem baru i kwasem siarkowym, mają szerokie zastosowanie. Na przykład, w przemyśle chemicznym, reakcje tego typu są wykorzystywane do neutralizacji ścieków kwasowych lub zasadowych, aby spełnić normy środowiskowe przed ich odprowadzeniem. Kontrola pH jest kluczowa w wielu procesach przemysłowych, a reakcje zobojętniania stanowią podstawę wielu z tych procesów.
W analizie chemicznej, miareczkowanie kwasowo-zasadowe, które opiera się na reakcjach zobojętniania, jest powszechnie stosowaną metodą do określania stężenia kwasów lub zasad w roztworach. Wykorzystuje się wskaźniki pH, które zmieniają kolor w zależności od pH roztworu, aby zidentyfikować punkt równoważnikowy, czyli moment, w którym kwas i zasada zostały całkowicie zneutralizowane.
Formowanie osadu, takie jak tworzenie się siarczanu baru, jest użyteczne w analizie jakościowej do identyfikacji obecności jonów baru lub jonów siarczanowych w roztworze. Dodanie roztworu zawierającego jony siarczanowe do roztworu zawierającego jony baru spowoduje utworzenie się białego osadu BaSO₄, co potwierdza obecność obu jonów.
Podsumowanie
Zrozumienie równań jonowych – pełnych i skróconych – jest kluczowe dla zrozumienia reakcji zachodzących w roztworach wodnych. Pozwala to na przewidywanie produktów reakcji i identyfikację jonów biorących w nich udział. Reakcja pomiędzy wodorotlenkiem baru i kwasem siarkowym jest doskonałym przykładem, który ilustruje te koncepcje.
Zapamiętaj, że kluczem jest poprawne zbilansowanie równania cząsteczkowego, rozpisanie rozpuszczalnych związków na jony w równaniu jonowym pełnym i wyeliminowanie jonów widzów, aby otrzymać równanie jonowe skrócone. Te kroki pozwolą na dokładne przedstawienie rzeczywistych zmian chemicznych zachodzących w roztworze.