Cześć! Dzisiaj zajmiemy się porównywaniem temperatur wrzenia różnych związków chemicznych.
Musimy zrozumieć, jakie siły działają między cząsteczkami.
Siły międzycząsteczkowe
To oddziaływania, które utrzymują cząsteczki blisko siebie.
Silniejsze oddziaływania oznaczają wyższą temperaturę wrzenia.
Rodzaje sił
Istnieją różne rodzaje sił, od najsłabszych do najsilniejszych.
Zaczniemy od sił Van der Waalsa.
To słabe oddziaływania wynikające z chwilowych nierównomierności w rozkładzie elektronów.
Następnie mamy oddziaływania dipol-dipol.
Występują w cząsteczkach polarnych, gdzie występuje trwały dipol elektryczny.
Najsilniejsze są wiązania wodorowe.
Tworzą się, gdy atom wodoru połączony z atomem silnie elektroujemnym (np. tlen, azot, fluor) oddziałuje z innym atomem silnie elektroujemnym.
Wiązania wodorowe są bardzo ważne dla właściwości wody!
Czynniki wpływające na temperaturę wrzenia
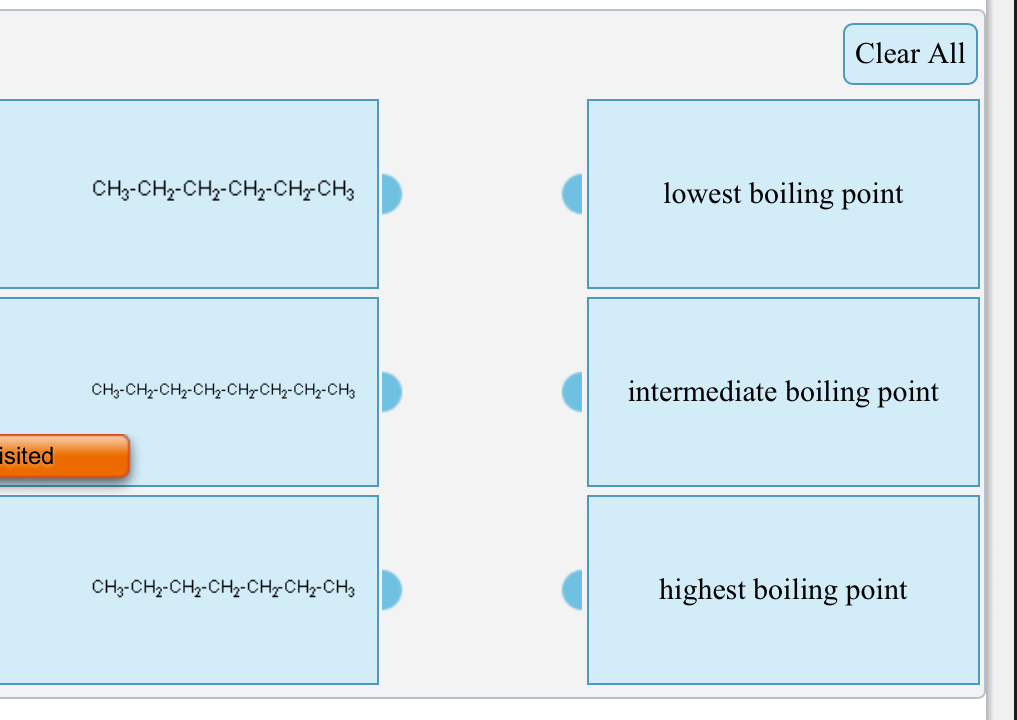
Oprócz rodzaju sił, na temperaturę wrzenia wpływa również masa molowa.
Im większa masa molowa, tym wyższa temperatura wrzenia (zazwyczaj).
Wynika to z większej liczby elektronów, co zwiększa siły Van der Waalsa.
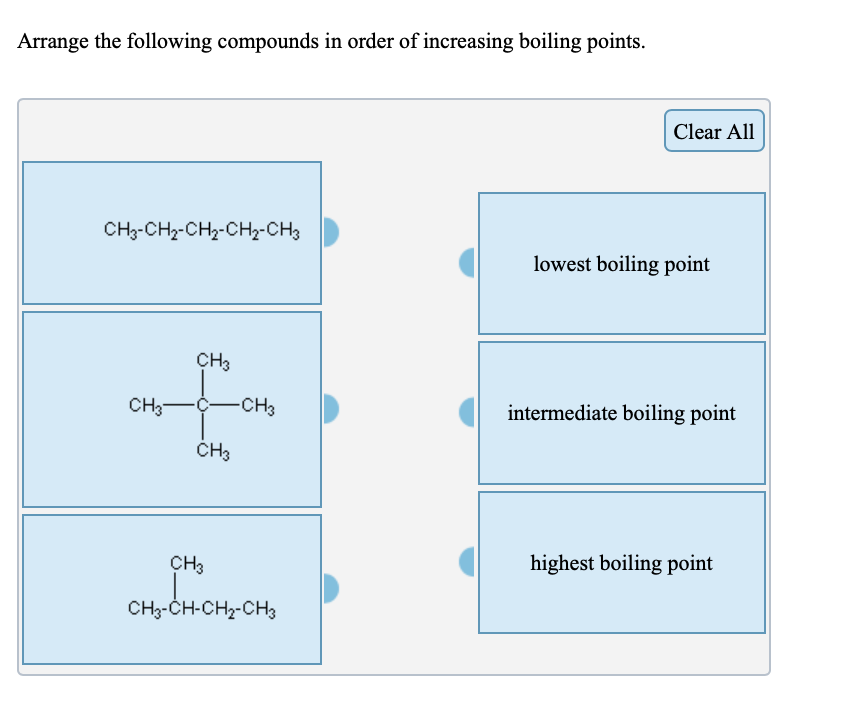
Kolejny czynnik to kształt cząsteczki.
Cząsteczki bardziej rozgałęzione mają mniejszą powierzchnię kontaktu, więc słabsze siły międzycząsteczkowe i niższą temperaturę wrzenia.
Przykłady i ćwiczenia
Załóżmy, że mamy następujące związki: metan (CH4), etan (C2H6), propan (C3H8) i butan (C4H10).
Wszystkie to alkany, więc oddziałują ze sobą głównie siłami Van der Waalsa.
Wraz ze wzrostem liczby atomów węgla rośnie masa molowa.
Zatem, temperatura wrzenia rośnie w kolejności: metan, etan, propan, butan.
A teraz trudniejszy przykład: etanol (C2H5OH), dimetyloeter (CH3OCH3) i propan (C3H8).
Propan ma tylko siły Van der Waalsa.
Dimetyloeter ma oddziaływania dipol-dipol (jest polarny).
Etanol ma wiązania wodorowe (dzięki obecności grupy -OH).
Zatem, temperatura wrzenia rośnie w kolejności: propan, dimetyloeter, etanol.
Wiązania wodorowe w etanolu są najsilniejsze, więc ma on najwyższą temperaturę wrzenia.
Porównywanie związków
Najpierw sprawdź, jakie siły międzycząsteczkowe występują w każdym związku.
Potem porównaj masy molowe.
Na koniec, weź pod uwagę kształt cząsteczki.
Zadanie:
Uporządkuj następujące związki w kolejności rosnącej temperatury wrzenia: woda (H2O), metanol (CH3OH), etan (C2H6).
Woda tworzy silne wiązania wodorowe.
Metanol też tworzy wiązania wodorowe, ale słabsze niż woda, bo ma większą część niepolarną.
Etan ma tylko słabe siły Van der Waalsa.
Odp: etan, metanol, woda.
Praktyczne zastosowania
Znajomość temperatur wrzenia jest ważna w wielu dziedzinach.
W przemyśle chemicznym, pomaga w procesach destylacji i separacji różnych substancji.
W farmacji, wpływa na stabilność i sposób podawania leków.
W inżynierii materiałowej, pomaga w projektowaniu tworzyw sztucznych i innych materiałów o odpowiednich właściwościach.
Na przykład, podczas destylacji ropy naftowej wykorzystuje się różnice w temperaturach wrzenia różnych frakcji węglowodorów, aby oddzielić benzynę, naftę, olej napędowy i inne produkty.
Podsumowując, aby uporządkować związki w kolejności rosnącej temperatury wrzenia, musimy wziąć pod uwagę siły międzycząsteczkowe, masę molową i kształt cząsteczki. Im silniejsze siły i większa masa molowa, tym wyższa temperatura wrzenia.
Powodzenia w dalszej nauce chemii!