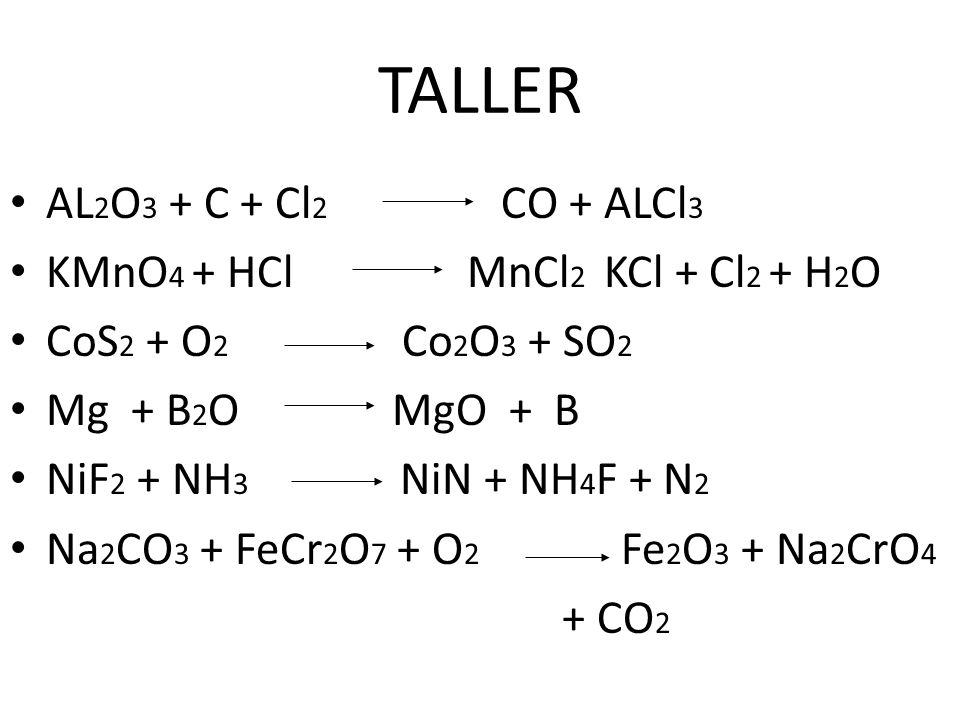
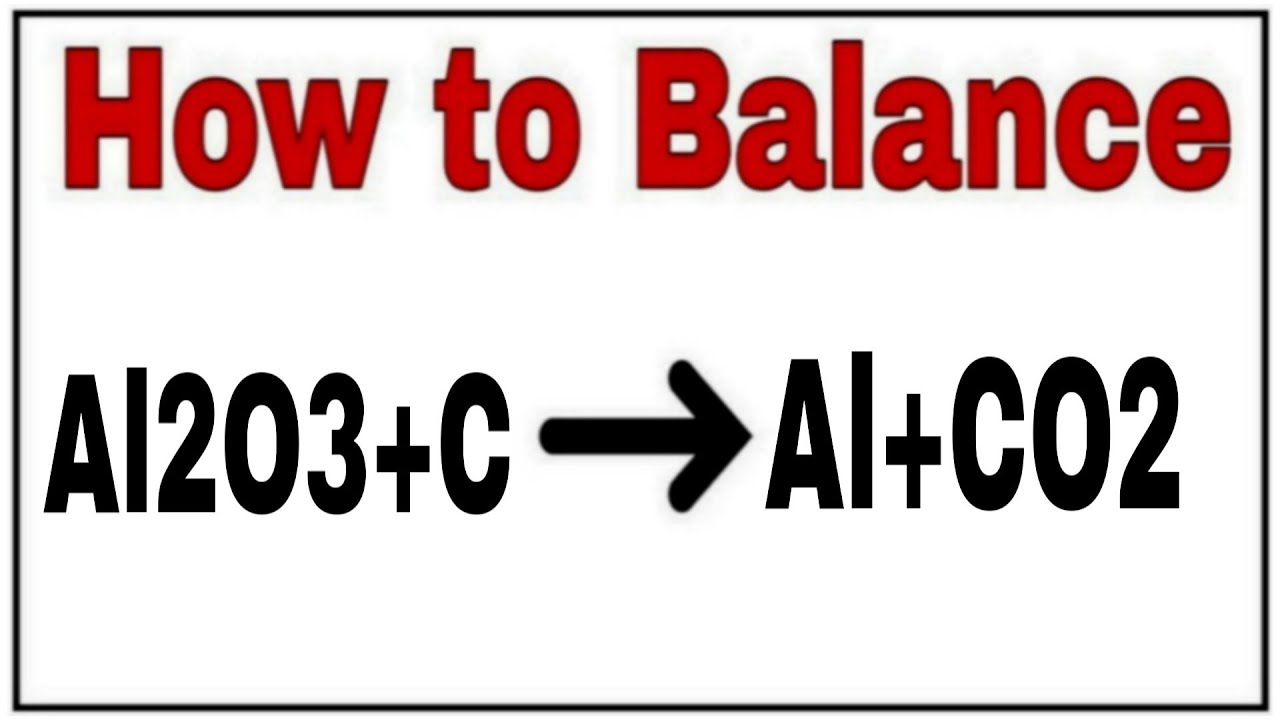
Wyobraź sobie, że budujesz z klocków LEGO. Mamy kilka różnych klocków, które w magiczny sposób możemy połączyć, tworząc coś zupełnie nowego. W naszym chemicznym świecie, tymi klockami są pierwiastki i związki chemiczne: Al2O3 (tlenek glinu), C (węgiel), Cl2 (chlor), CO (tlenek węgla) i AlCl3 (chlorek glinu). Zobaczymy, jak z tych klocków można zbudować ciekawy proces!
Glin, Tlen i Magiczna Skała: Al2O3
Zacznijmy od Al2O3, czyli tlenku glinu. Wyobraź sobie, że to bardzo twarda skała. Glin (Al) i tlen (O) mocno się ze sobą trzymają. Tlenek glinu występuje naturalnie jako korund, a w jego odmianach znajdziemy rubiny i szafiry! Pomyśl o tych kamieniach szlachetnych – są bardzo twarde i odporne. Tlenek glinu ma podobne właściwości, dlatego wykorzystuje się go na przykład do produkcji materiałów ściernych, takich jak papier ścierny. Widzisz ten papier ścierny? Dzięki tlenkowi glinu możesz szlifować różne powierzchnie!
Al2O3 ma postać białego, sypkiego proszku. Wyobraź sobie śnieg – tylko dużo trwalszy i odporniejszy na temperaturę. Tlenek glinu jest bardzo odporny na wysokie temperatury, więc świetnie sprawdza się jako materiał izolacyjny.
Węgiel: C, Czarny Diament i Grafit
Teraz spójrzmy na C, czyli węgiel. To bardzo wszechstronny klocek. Może występować w różnych postaciach. Wyobraź sobie diament – najtwardszy materiał na Ziemi, czysty węgiel. A teraz pomyśl o graficie w ołówku – miękki i śliski, też węgiel! To pokazuje, jak różne mogą być właściwości tego samego pierwiastka, w zależności od tego, jak są ułożone jego atomy.
W naszym przypadku, węgiel pełni rolę reduktora. Wyobraź sobie małego pomocnika, który zabiera tlen innym związkom.
Chlor: Cl2, Gaz o Ostrym Zapachu
Przechodzimy do Cl2, czyli chloru. To gaz o ostrym, duszącym zapachu. Pamiętasz zapach basenu? To właśnie chlor! (A dokładnie związki chloru, które powstają w reakcji z wodą). Chlor jest silnym utleniaczem i ma właściwości dezynfekujące. Wyobraź sobie, że chlor to mały żołnierz, który walczy z bakteriami i wirusami.
Musimy bardzo uważać z chlorem, ponieważ jest toksyczny. W laboratorium używa się go tylko pod ścisłą kontrolą.
Tlenek Węgla: CO, Cichy Zabójca
Kolejny związek to CO, czyli tlenek węgla. Nazywany jest "cichym zabójcą", ponieważ jest bezbarwny i bezwonny, a bardzo trujący. Powstaje podczas niepełnego spalania węgla. Wyobraź sobie źle działający piec węglowy – to potencjalne źródło tlenku węgla. Dlatego tak ważne są czujniki czadu!
Chlorek Glinu: AlCl3, Biały Proszek
Na koniec AlCl3, czyli chlorek glinu. To biały, higroskopijny proszek (czyli chłonie wilgoć z powietrza). Wyobraź sobie sól kuchenną, tylko że zamiast sodu (Na) mamy glin (Al) i zamiast jednego atomu chloru, mamy ich aż trzy! Chlorek glinu jest bardzo ważnym katalizatorem w wielu reakcjach chemicznych. Katalizator to taka osoba, która przyspiesza reakcję chemiczną, ale sama w niej nie uczestniczy.
Można go porównać do osoby, która zagrzewa do działania i mobilizuje innych. W przemyśle chlorek glinu wykorzystuje się na przykład do produkcji barwników i leków.
Wielka Reakcja: Produkcja Glinu
Teraz połączmy te klocki w całość. Możemy wykorzystać je w procesie produkcji glinu. Glin otrzymuje się z tlenku glinu (Al2O3) w procesie elektrolizy. Ale zanim to nastąpi, tlenek glinu rozpuszcza się w roztopionym kriolicie, a węgiel (C) i chlor (Cl2) oraz tlenek węgla (CO) oraz chlorek glinu (AlCl3) odgrywają rolę w procesach pomocniczych lub alternatywnych metodach otrzymywania glinu lub jego związków.
Wyobraź sobie wielką fabrykę, gdzie w specjalnych piecach zachodzi reakcja. Al2O3 jest ogrzewany w obecności węgla, który "zabiera" tlen od glinu. W wyniku tego powstaje czysty glin (Al) oraz tlenek węgla (CO). Cały proces jest bardzo skomplikowany i wymaga wysokiej temperatury i specjalnych warunków.
Ten proces jest jak magiczna transformacja, gdzie z twardej skały tlenku glinu powstaje lekki i wytrzymały metal – glin, który wykorzystujemy na co dzień w puszkach, samolotach i wielu innych przedmiotach!
Mam nadzieję, że dzięki temu porównaniu do klocków LEGO i obrazowym przykładom, łatwiej zrozumiesz, czym są te związki chemiczne i jaką rolę odgrywają w chemii!